Difference between revisions of "Computational Regulatory Genomics"
| Line 4: | Line 4: | ||
[[File:Beer_Michael_small.jpg]] [[File:EncodeNatureGraphic_small.png]] [[File:Beer_lab_plate_art_small.jpg]] | [[File:Beer_Michael_small.jpg]] [[File:EncodeNatureGraphic_small.png]] [[File:Beer_lab_plate_art_small.jpg]] | ||
| − | <h3>Research Interests: </h3> The ultimate goal of our research is to understand how regulatory | + | <h3>Research Interests: </h3> The ultimate goal of our research is to understand how gene regulatory information is encoded in genomic DNA sequence. |
We have recently made significant progress in understanding how DNA sequence features control cell-type specific mammalian enhancer activity by using kmer-based SVM machine learning approaches. For details, see: | We have recently made significant progress in understanding how DNA sequence features control cell-type specific mammalian enhancer activity by using kmer-based SVM machine learning approaches. For details, see: | ||
Revision as of 03:41, 2 December 2013
Welcome to the Beer Lab!
Research Interests:
The ultimate goal of our research is to understand how gene regulatory information is encoded in genomic DNA sequence.
We have recently made significant progress in understanding how DNA sequence features control cell-type specific mammalian enhancer activity by using kmer-based SVM machine learning approaches. For details, see:
- Mammalian Enhancer Prediction. Lee D, Beer MA. 2014. Genome Analysis: Current Procedures and Applications. Horizon Press (in press)
- Robust k-mer Frequency Estimation Using Gapped k-mers. Ghandi M, Mohammad-Noori M, and Beer MA. 2013. Journal of Mathematical Biology. (Epub ahead of print)
- kmer-SVM: a web server for identifying predictive regulatory sequence features in genomic datasets. Fletez-Brant C*, Lee D*, McCallion AS and Beer MA. 2013. Nucleic Acids Research 41: W544–W556.
- Integration of ChIP-seq and Machine Learning Reveals Enhancers and a Predictive Regulatory Sequence Vocabulary in Melanocytes. Gorkin DU, Lee D, Reed X, Fletez-Brant C, Blessling SL, Loftus SK, Beer MA, Pavan WJ, and McCallion AS. 2012. Genome Research 22:2290-2301.
- Discriminative prediction of mammalian enhancers from DNA sequence. Lee D, Karchin R, and Beer MA. 2011. Genome Research 21:2167-2180.
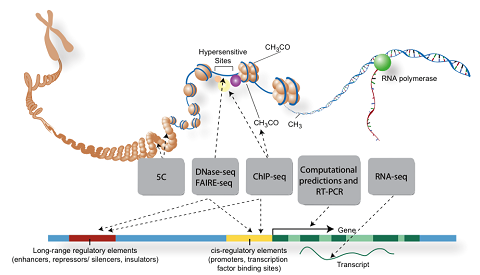
Our work uses functional genomics DNase-seq, ChIP-seq, RNA-seq, and chromatin state data to computationally identify combinations of transcription factor binding sites which operate to define the activity of cell-type specific enhancers. We are currently focused on:
- improving this methodology by including more general sequence features and constraints
- predicting the impact of SNPs on enhancer activity (delta-SVM) and GWAS association for specific diseases
- experimentally assessing the predicted impact of regulatory element mutation in mammalian cells
- systematically determining regulatory element logic from ENCODE human and mouse data
- using this sequence based regulatory code to assess common modes of regulatory element evolution and variation
We are located in the McKusick-Nathans Institute for Genetic Medicine, and the Department of Biomedical Engineering, which has long been a leader in the development of rigorous quantitative modeling of biological systems, and is a natural home for graduate studies in Bioinformatics and Computational Biology at Johns Hopkins, including research in Genomics, Systems Biology, Machine Learning, and Network Modeling.